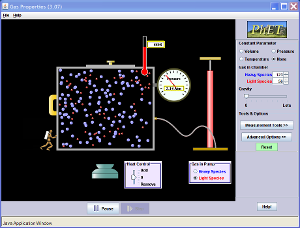
5.7 and 5.8 Starter answers
· What are the 6 processes shown by the arrows?
1a
· Particles in a solid are strongly bonded to each other so their particles are held in a fixed, regular pattern and can not move
· The bonds between particles in liquids and gases are weaker and therefore their particles can move relative to each other
1b
· The particles in solids and liquids are closely packed and they are therefore incompressible
· The particles in a gas are very widely spaced and the forces between them are very weak so they can spread out to fill their container
Boiling
· Boiling occurs when you heat a liquid until the average energy of the particles is great enough for them to turn into a gas
· Boiling occurs at a fixed temperature called the boiling point
· Boiling occurs throughout a liquid
· It is a fast process
·
Evaporation
· Evaporation occurs when a liquid is left open to the air
· Only particles at the surface of the liquid that have enough energy can escape the liquid into the air
· Evaporation occurs for a range of temperatures; high temperatures increase evaporation, low temperatures decrease evaporation
· Evaporation only occurs from the surface of a liquid
· It is a slower process
· Because it removes the most energetic particles from a liquid the average energy of the remaining particles is decreased and the liquid cools down